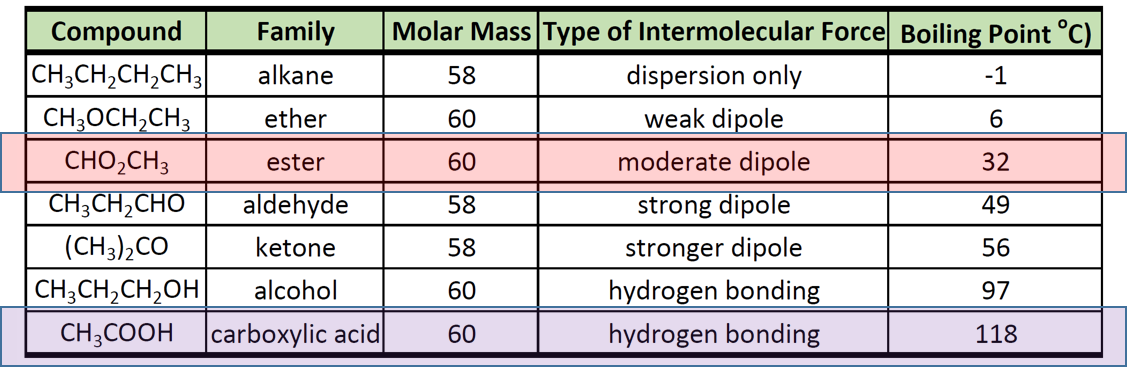
Boiling Point of Ester and Carboxylic Acid
Linear Structure Formula -InChI Key. Since 2010 diesel fuel may contain up to 7 vol fatty acid methyl ester FAME in Europe to meet biofuels.
Ch105 Chapter 9 Organic Compounds Of Oxygen Chemistry
However as phosphoric acid has further -OH functionalities triphosphates may also be formed.

. Materials Organic ligands for MOF materials Carboxylic MOF ligands Halogen series MOF ligands of nitrogenous. How might you account for this trend. Carboxylic acid any of a class of organic compounds in which a carbon C atom is bonded to an oxygen O atom by a double bond and to a hydroxyl group OH by a single bond.
A fourth bond links the carbon atom to a hydrogen H atom or to some other univalent combining group. A dicarboxylic acid is an organic compound containing two carboxyl functional groups COOH. As with carboxylic acids two phosphoric acid molecules may combine with the loss of water to form a di phosphate ester also referred to as pyrophosphate.
3551C at 760 mmHg. The carboxyl COOH group is so-named because of the carbonyl group CO and hydroxyl group. The boiling range is generally 160360C 320680F.
1-Butanol bp 1175C 2-Butanol bp 935C 2-Methyl-2-propanol bp 322C. Salts and esters of valeric acid are known. Carboxylic acids and carboxylates prevent free water in the gasoline from rusting or corroding.
The general molecular formula for dicarboxylic acids can be written as HO 2 CRCO 2 H where R can be aliphatic or aromatic. Acid Dyes Azoic Dyes Basic Dyes Direct Dyes Disperse Dyes Dye Intermediates Mordant Dyes Oil Dyes Other Stains and Dyes Synthetic Reagents Acids and. Valeric acid or pentanoic acid is a straight-chain alkyl carboxylic acid with the chemical formula CH 3 CH 2 3 COOHLike other low-molecular-weight carboxylic acids it has an unpleasant odorIt is found in the perennial flowering plant Valeriana officinalis from which it gets its nameIts primary use is in the synthesis of its esters.
Hydrolysis of ester gives carboxylic acid and. The following data for isomeric four-carbon alcohols show that there is a decrease in boiling point with increasing substitution of the OH-bearing carbon. In general dicarboxylic acids show similar chemical behavior and reactivity to monocarboxylic acidsDicarboxylic acids are also used in the preparation of.
2 is similar in composition to fuel oil no.
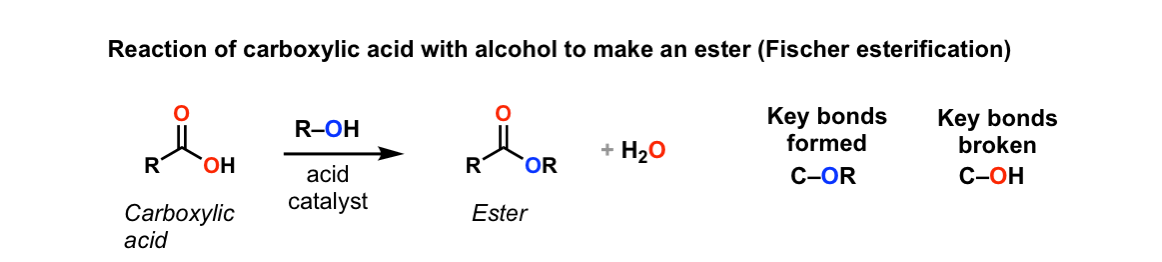
Conversion Of Carboxylic Acids To Esters Using Acid And Alcohols Fischer Esterification Master Organic Chemistry

Physical Properties Of Carboxylic Acids Youtube

Carboxylic Acid Have Higher Boiling Points Than Aldehydes Ketones And Even Alcohol Of Youtube

Comments
Post a Comment